Description
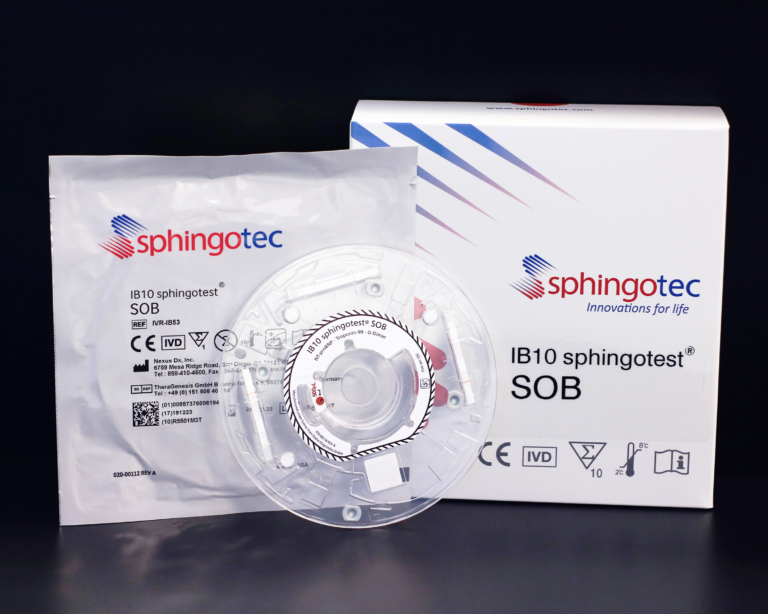
IB10 Biorigins® SOB (Shortness of Breath), is a rapid point-of-care (POC) immunoassay for the in vitro quantitative determination of Cardiac Troponin I (cTnI), N-terminal pro-brain natriuretic peptide (NT-proBNP) and D-Dimer in lithium heparin human whole blood and plasma. The IB10 Biorigins SOB is intended for use in conjunction with the IB10 Analyzer and provides quantitative results in 20 minutes.
The SOB (Shortness of Breath) test panel is intended as an aid in the differential diagnosis and prognostic assessment of patients with symptoms of chest pain, typically accompanied by respiratory distress. Individually or in conjunction with each other, these markers: aid in the diagnosis of myocardial infarction (MI), aid in the risk stratification of patients with acute coronary syndrome (ACS) including prediction of the likelihood of developing heart failure (HF), aid in the diagnosis, assessment of severity and likelihood of survival in HF, and aid in determining the probability of rule-out of patients presenting with clinical symptoms of venous thromboembolism (VTE) including pulmonary embolism (PE) and deep vein thrombosis (DVT).
This test is designed for professional use only and may be used in hospital central laboratories and in alternate care settings such as emergency departments, critical care units, and other sites where near patient testing is practiced.
Disclaimer:
Products based on the IB10 technology ( San Diego, CA, USA) described are CE-IVD-marked and hence certified and approved for use in the European Economic Area (EEA) only. Human diagnostic use of such products may be subject to local regulations.
Caution – The information contained in this communication does not constitute nor imply an offer to sell or transfer any product based on the IB10 technology as in vitro diagnostic (IVD) product in the United States of America or Canada. No product based on the IB10 technology is currently available for sale as an IVD product in the United States of America or Canada. The analytical and clinical performance characteristics in compliance with U.S. FDA medical device regulations of any IB10 product may be sold at some future point in time in the U.S., have not yet been established.
Product is sold only in Africa.
- “Products and Price List” of the Supply and Service Agreement.
- Defined terms used herein and not otherwise defined shall have the meanings given to them in the Agreement.
- All unmodified and remaining terms and conditions of the Agreement are hereby ratified and confirmed and shall remain in full force and effect.
- This agreement shall be governed by and construed in accordance with the laws of the (Name of Country), without regard to the principles of conflict of laws.




There are no reviews yet.